electronic configuration of zinc 2+|What is the electron configuration for Zn^2+? Chemistry Q&A : Tuguegarao 1 Answer. The electron configuration of Zn2+ is 1s22s22p63s23p63d10. The atomic number of zinc is 30, which means that all zinc atoms have 30 protons in . Ask the Tropers is for: • General questions about the wiki, how it works, and how to do things. • Reports of problems with wiki articles, or requests for help with wiki articles. • Reports of misbehavior or abuse by other tropers. Ask the Tropers is not for: • Help identifying a trope. See TropeFinder. • Help identifying a work. See .Enter Piso WiFi Pause! Simply access the Piso WiFi web management interface (usually at 10.0.0.1) and click “Pause Time.” Voila! Your internet clock stops ticking, preserving your precious minutes until you resume. Think of it as a time capsule for your data – safely tucked away until you’re back online. Why Should You Use Piso WiFi .
electronic configuration of zinc 2+,The configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom. This makes it easier to understand and predict.electronic configuration of zinc 2+ Mar 23, 2023
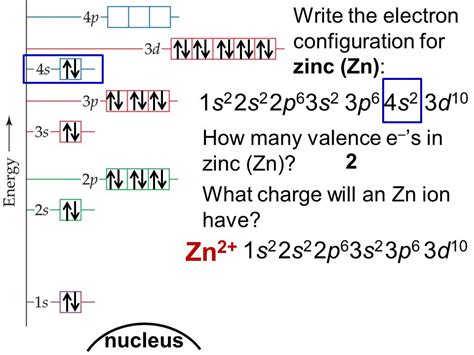
The electronic configuration of Zinc is 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 3 d 10 4 s 2. Electronic configuration of Zinc ion Zn 2 + : Zn loses its two electrons and results in the formation of . 1 Answer. The electron configuration of Zn2+ is 1s22s22p63s23p63d10. The atomic number of zinc is 30, which means that all zinc atoms have 30 protons in .
Determine the electron configuration of ions. Justify the observed charge of ions to their electronic configuration. Define paramagnetism and diamagnetism. Justify the .
The electron configuration of a neutral zinc atom is $$1s^{2}2s^{2}2p^{6}3s^{2}3p^{6}3d^{10}4s^{2}$$. The $$Zn^{2+}$$ ion has lost two .
What is the electron configuration for Zn^2+? Chemistry Q&A Zinc Electronic Configuration is: 1s² 2s² 2p⁶ 3s² 3p⁶ 4s2 3d¹⁰. In this article, we will study how electrons are arranged in different shells and subshells in the Zinc .The first electron has the same four quantum numbers as the hydrogen atom electron ( n = 1, l = 0, ml = 0, ms = + 1 2 ). The second electron also goes into the 1 s orbital and fills that orbital. The second electron . Zinc Electron Configuration: Zinc is a chemical element which has a chemical symbol Zn. The atomic number of zinc is 30. It is the first element of a twelfth group of the periodic table. In some .
Zinc is a member of group 12, so it should have a charge of 2+, and thus loses only the two electrons in its s orbital. . Oxygen, for example, has the electron configuration 1s 2 2s 2 2p 4, whereas the oxygen anion .
Add an electron to the anion electron configuration. For example, the ground state electronic configuration of chlorine is 1s²2s²2p⁶3s²3p⁵. For Cl −, it will be 1s²2s²2p⁶3s²3p⁶. Remove the outermost electrons in the cation, e.g. electron configuration for Mg 2+ will be 1s²2s²2p⁶.

For example, the electronic configuration of sulfur can be written as [Ne] 3s 2 3p 4, since Neon has an electronic configuration of 1s 2 2s 2 2p 6. Electronic Configuration of First 30 Elements with Atomic .
The atomic number of Zinc (Zn) is 30. Therefore, the electronic configuration of Zinc can be represented as: 1s2 2s2 2p6 3s2 3p6 4s2 3d10. Here, the first shell contains 2 electrons (1s²), the second shell contains 8 electrons (2s² 2p⁶), the third shell contains 18 electrons (3s² 3p⁶), and the fourth shell contains 2 electrons in the s .
In the case of first row transition metals, the electron configuration would simply be [Ar] 4s x 3d x. The energy level, "n", can be determined based on the periodic table, simply by looking at the row number in which the element is in. However, there is an exception for the d-block and f-block, in which the energy level, "n" for the d block is .This page shows the electron configurations of the neutral gaseous atoms in their ground states. For each atom the subshells are given first in concise form, then with all subshells written out, followed by the number of electrons per shell. For phosphorus (element 15) as an example, the concise form is [Ne] 3s 2 3p 3.
The electron configuration turns out to be 4s 2, 3d 1. It's actually 4s 2, 3d 1 or if you prefer 3d 1, 4s 2 once again with argon in front of it. . We're adding one more, writing one more .
Orbital Diagram, electron configuration, and the noble gas notation for a zinc (Zn) atom.
electronic configuration of zinc 2+ What is the electron configuration for Zn^2+? Chemistry Q&AIf an ion has a negative charge, we have to add the electrons from the neutral electronic configuration of the element. The given ion – Zn 2+. 2+ charge indicates substraction of two electrons from neutral electronic configuration. Zn electronic configuration – 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10. We have to remove two electrons from zinc .The atomic number of zinc is $$30$$, which means that the neutral atom has equal numbers of protons and electrons, so a neutral atom of zinc would have $$30$$ electrons. The electron configuration of a neutral zinc atom is $$1s^{2}2s^{2}2p^{6}3s^{2}3p^{6}3d^{10}4s^{2}$$.

In this case, 2+2+6+2+6+2+10+6+2+1= 39 and Z=39, so the answer is correct. A slightly more complicated example is the electron configuration of bismuth (symbolized Bi, with Z = 83). The periodic .The cell representation of the given reaction is:Zn (s) + Cu 2+ → Zn 2+ + Cu (s) View Solution. Click here:point_up_2:to get an answer to your question :writing_hand:write electronic configuration for cuquad cu 2 quad zn 2 quad. The common shorthand notation is to refer to the noble gas core, rather than write out the entire configuration. For example, the configuration of magnesium could be written [Ne]3s 2, rather than . Zinc Electron Configuration. The Mg 2+ and Zn 2+ ions are of the same size. Zinc is the 24th most abundant element present in the Earth’s crust. It has five stable isotopes. The common zinc ore is sphalerite or zinc blende, which is a zinc sulfide mineral. The largest working lodes of Zinc are in Asia, Australia, and the United States.
Both of the configurations have the correct numbers of electrons in each orbital, it is just a matter of how the electronic configuration notation is written (here is an explanation why). Therefore we have 1s 2 2s 2 2p 6 3s 2 3p 6 3d 6 4s 2. For the Fe2+ ion we remove two electrons from 4s2 leaving us with: 1s 2 2s 2 2p 6 3s 2 3p 6 3d 6
Electron atomic and molecular orbitals A Bohr diagram of lithium. In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. For example, the electron configuration of the neon atom is 1s 2 2s 2 2p 6, meaning that the 1s, 2s, and . The electronic configuration of cations is assigned by removing electrons first in the outermost p orbital, followed by the s orbital and finally the d orbitals (if any more electrons need to be removed). For instance, the ground state electronic configuration of calcium (Z=20) is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2. For example, the electron configurations of the transition metals chromium (Cr) and copper (Cu), are not those we would expect. Rather, Cr and Cu take on half-filled and fully-filled 3d configurations. The electron configuration of chromium (Cr) includes a half-filled 3d subshell. Cr: 1s 2 2s 2 2p 6 3 s 2 3p 6 4s 1 3d 5
electronic configuration of zinc 2+|What is the electron configuration for Zn^2+? Chemistry Q&A
PH0 · Zinc Electronic Configuration and Distribution in Shells
PH1 · Zinc Electron Configuration (Zn) with Orbital Diagram
PH2 · What is the electron configuration for Zn^2+? Chemistry Q&A
PH3 · What is the electron configuration for Zn^2+? Chemistry Q&A
PH4 · What is the electron configuration for Zn2+?
PH5 · What is the electron configuration Zn^{2+}?
PH6 · Electron Configuration for Zn and Zn2+ (Zinc and Zinc ion)
PH7 · Electron Configuration Chart of All Elements (Full Chart)
PH8 · Complete Electron Configuration for Zinc (Zn, Zn2+ ion)
PH9 · 7.4: Electron Configurations of Ions
PH10 · 3.1: Electron Configurations